This cutting-edge blended course focuses on how to develop new medical devices and maintain them in an organisation where design control requirements apply. It covers both the EU and US requirements. You learn the theory first during the online course, and then apply that theory during the two live virtual classroom sessions.
| Course length | approx. 16-31 hours (24 CPD points) |
| Next classroom sessions |
1-2 Oct 2025 |
Free
Average completion time 1-2 hours
Total video duration 0.5 hours
Number of quizzes 5
Final exam
30 day access to the course
Digital course companion
€ 399 Average completion time 9-24 hours
Total video duration 6 hours
CPD points 17
Number of quizzes 35
Final exam
Course certificate
Ask the instructor
6 months access to the course and instructor
Digital course companion
€ 499 Average completion time 9-24 hours
Total video duration 6 hours
CPD points 17
Number of quizzes 35
Final exam
Course certificate
Ask the instructor
Lifetime access to the course and instructor
Digital course companion
YOU ARE HERE
€ 999 Average completion time 16-31 hours
Total video duration 6 hours
CPD points 24
Number of quizzes 35
Final exam
Course certificate
Ask the instructor
Lifetime access to the course and instructor
Digital course companion
Classroom sessions (2 * 3.5 hours)
Templates
Tailored training to meet your company's needs
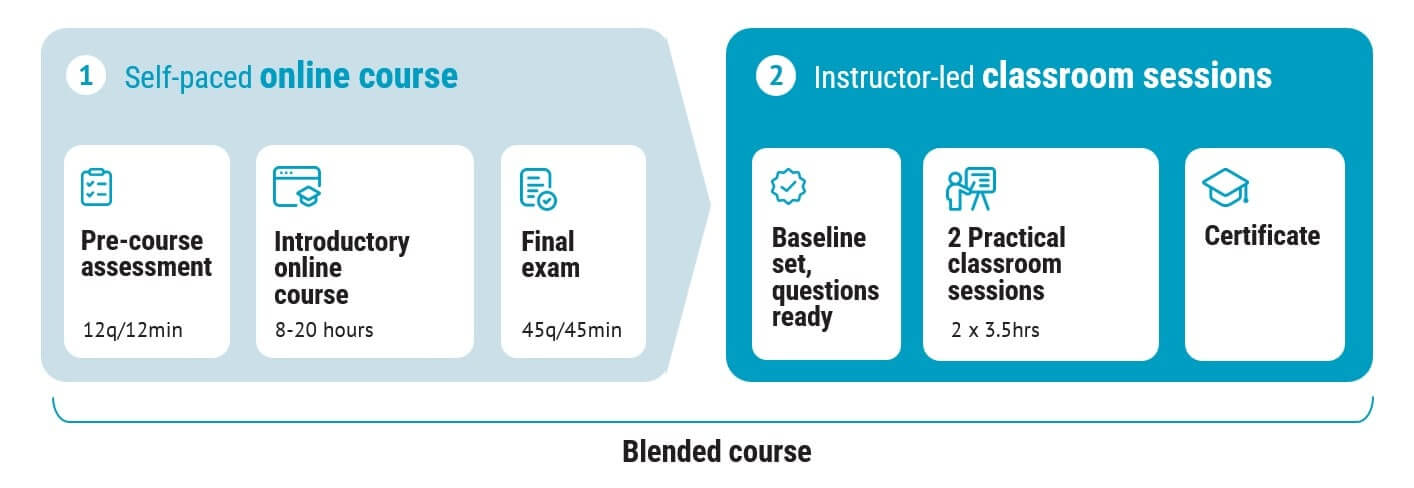
This course consists of two parts:
1. The Introduction to Design Control for Medical Devices online course
2. Two half day live virtual classroom sessions faciliated by an instructor
First, you complete the popular introductory design control online course. It covers topics such as the product development process, user needs and design input, design verification and design validation, design transfer, introductions to risk management, and usability engineering.
You can start the online course immediately after enrolment and you must successfully complete the final exam prior to the classroom sessions. If you have already completed the online course, you can register for the classroom sessions only.
The unique, highly interactive live virtual classroom sessions are facilitated by Peter Sebelius, Helena Hjälmefjord or Claus Rømer Andersen. During the sessions, you get to apply the theory you have learnt via case studies, workshops, and discussions. There is plenty of time for Q&As, and with a maximum of 8 people per classroom session, all participants have the opportunity to learn first hand from an industry expert.
A downloadable course companion and time-saving templates are included in the blended course.
We recommend this course to those who are new in the field, but also to professionals involved in the development of medical devices, such as quality assurance, project management, and design engineering, or those involved in R&D and product development teams.
Our blended learning courses combine the best of both worlds: online training and two half-day live virtual classroom sessions afterwards.
The online training allows for the flexibility in the way you learn, so that you can learn in a way which suits you best. You can access it whenever and wherever you want, and you can stop, start, and repeat the lessons and quizzes as often as you would like. To help maximise your learning, you can check your knowledge and understanding every step of the way by taking the assessment test beforehand, as well as the checkpoint quizzes that are found throughout the course. Ultimately, you will take a final exam at the end of the course.
The classroom sessions are highly interactive and focuses on applying the theory you have learnt during the online course with the guidance of the expert instructor. There are real life case scenarios to work through and plenty of time for discussions and questions.

Peter Sebelius is a highly esteemed trainer, consultant and entrepreneur in the medical device industry. He is a member of the Joint Working Group that is revising the ISO 13485 and ISO 14971 standards.
He has vast ‘hands on’ experience, having developed, amongst other things, a mechanical chest compression device and an ex vivo perfusion machine for lungs. He has received numerous awards including the Great Design Award and the title “This year’s specialist” by Veckans affärer.

Helena Hjälmefjord has extensive experience within the medical device industry, Class I to Class III devices and In-Vitro diagnostics; as well as with allografts (human tissue). Amongst others she has worked as project leader, quality manager as well as design control and regulatory assurance lead. Helena has worked in both small companies as well as large international companies. Since 2014 she works through her own consultant company, Fjord Consulting.
Helena’s motivation is getting customers to understand the importance and benefits of the regulations that are placed on their medical device products and related processes.

Claus Rømer Andersen is an accomplished trainer, consultant and facilitator in the medical device industry. With a background in electrical engineering, he has worked with regulatory navigation, approval management, device testing throughout his whole career.
He is recognized as having a pragmatic and solution oriented approach to helping development teams focus on relevant issues throughout the entire product life-cycle.
Design control can be made incredibly abstract if there is too much focus on the intangible requirements instead of how to actually do the work.
This course is very pragmatic in its approach, and it covers both how to find and interpret requirements as well as how to implement them.
We understand the importance of retaining knowledge beyond the course duration. That’s why we provide a digital course companion of key concepts accompanied by relevant images and helpful flowcharts.
The PDF format enables you to take notes and personalize the material according to your learning preferences.
Our instructors are passionate about what they do, bringing their expertise and love for the subject into the learning experience.
Participate in active learning by asking questions directly to the instructor, taking part in group workshops and discussions. This allows you to seek clarification, share insights, apply and transfer knowledge.
The online course comprises a mixture of pre-recorded videos, quizzes, and a final exam. It is self-paced and flexible so that you can start, stop, and repeat as much as you like. You will have lifetime access to the online course (conditions apply).
The Introduction to Design Control for Medical Devices online course consists of 20 lessons which are outlined below.
You will attend these two half-day live virtual instructor-led classroom sessions via Zoom. The focus is on practicing the new skills, knowledge, and understanding learnt during the online course, in a relaxed environment, with the guidance of an expert instructor. There are real–life case scenarios to work through, and plenty of time for discussions and questions.
The live virtual classes will cover the product development process and design control, how to write requirements, and the most common pitfalls in medical device development, as well as how to avoid them.
The maximum number of people in a classroom session is 8.
Included is a digital course companion and the following premium time-saving templates:
The blended course consists of two parts: the online course, and two half day live virtual classroom sessions.
The online course is made up of carefully scripted pre-recorded videos in order to maximise your learning. The live virtual classroom sessions are face-to-face sessions where you will be interacting with the instructor and the other course participants via Zoom. You are required to be visible on video when taking the classroom sessions.
Yes, you can.
The time spent on the course varies greatly. If you are a beginner or have very high ambitions, you will typically spend more time on it. If you are already experienced in the field, you will probably finish it more quickly. The online course takes 9-24 hours to finish on average.
You do not have to watch the whole course at once. In fact, you can pause, resume, and replay as often as you like.
The two half-day live virtual classroom sessions, take approximately 7 hours in total.
You will receive a course certificate after completing the final exam. The course certificate is available when you log in to your account. Go to “My Courses”, and scroll down. You will find it under “Your Course Certificates”.
After successfully completing the classroom session, you will receive another course certificate covering both the online course and the classroom session by email.
If you wish to buy 3 or more seats, we invite you to reach out using the contact form to discuss discount options. Please note, if there are more than 5 people from your organisation wising to take the course, consider arranging an in-house course. The price will be lower, and the course can be customised to your needs.
If you complete the final exam within 6 months of enrolment, you will have lifetime access to the online course (conditions apply). If you do not, your course access will expire after 6 months.
Yes, you can download a course companion within the online course. It contains a selection of key slides from the course and is in PDF format.
The maximum number of participants for the live virtual classroom session is 8.
There is no pass/fail score on the exam as such, but you are required to achieve 85% or more on the final exam to be allowed to attend the classroom session. The check-point quiz questions that are available during the online course will be a good model of what you will get on the final exam.
If you do not achieve 85%, the final exam can be reset for an administrative fee of 50 EUR, and you can take it again.
If you have already completed the online course and taken the final exam more than 3 weeks prior to the classroom sessions, and regardless of whether you have achieved >85%, we will reset the final exam for you for free.
You will need to retake the final exam by 3pm CET the day before the first classroom session. This is because we insist all participants have the information fresh in their mind in order to maximise the learning experience in the live virtual classroom sessions.
There are no pre-requisites for this course.
The instructor has conducted training in the field for many years and continuously participates in authoring standards such as the ISO 13485 and ISO 14971. This ensures the instructor has access to leading-edge knowledge and information, which is the highest qualification available for teaching the course.
We offer a number of payment methods including credit card, GooglePay, ApplePay, PayPal, wire transfer/invoice depending on the country you are buying from. If you cannot find your country on the list at checkout or you are not given wire transfer as a payment method, you can submit your order via our invoice order form.
Medical Device HQ is proud to be an accredited CPD provider, demonstrating our commitment to your continued professional development with high-quality training courses.
A CPD record showcases your professional growth, boosts confidence in your abilities, and can be presented to management for better career opportunities.
As a participant of a CPD certified course, you will receive a certificate awarding you CPD points following completion of the training.
Upon completion of this blended course, you are awarded 24 CPD points.
Your course certificate will display the exact number of CPD points. Read more.






We are naturally happy to hold courses on your premises. Simply contact us to discuss your requirements.
If you are already prepared to take a course, you can register for one of our upcoming blended courses. You’ll have 24 hours to cancel your reservation in case you can’t come.
IMPORTANT – The course will be associated with the account that the purchase is made from. Are you taking the course or is someone else?
Choose your course options below
Choose your course options below
IMPORTANT – The course will be associated with the account that the purchase is made from. Are you taking the course or is someone else?
Oops, I actually wanted to buy seats for several people. Take me to the right place.
Special launch offer: 349 299 EUR for the online plan & 449 349 EUR for the online lifetime plan.