
It’s been almost two years since the World Health Organization (WHO) declared that Covid-19 was no longer an international public health emergency. Since then, some things have gone back to “normal” (pre-pandemic) while other things have evolved and changed, perhaps permanently.
People are no longer asking what learning will be like after Covid but rather: What is the future of learning?
Before the Covid-19 pandemic hit, Medical Device HQ had delivered traditional classroom courses to businesses for over ten years. The last traditional classroom course we held before the pandemic was in February 2020.
At the time, multiple traditional classroom courses had already been cancelled. And what could have become a total disaster for the company, ended up being a surge in demand for our online courses and a rapid change in how our training courses were delivered. This resulted in organic growth by 27% during the year of the pandemic.
Just like many others did in response to the lockdown, we quickly changed a few courses to virtual, instructor-led training. But was this really going to be the long-term solution? Even before the pandemic, we had delivered online courses, as well as a rare type of course: blended, or hybrid courses.
“Blended learning involves leveraging multiple platforms to deliver training content to learners. Blending online theoretical content with a hands-on practical application or mentor-based instruction is an efficient, cost-effective delivery method for workforce training.”
(Lothridge, Karen; et al. (2013). “Blended learning: efficient, timely, and cost-effective”. Journal of Forensic Sciences. 45 (4): 407–416.)
In the period from June to December of 2020, Medical Device HQ delivered close to 50 blended courses, with around 400 participants. Since then, we have steadily delivered up to 100 blended courses every year. The participants do a pre-course assessment quiz, then attend an online course, and take a final exam qualifying them for a two half-day live virtual or F2F classroom sessions.
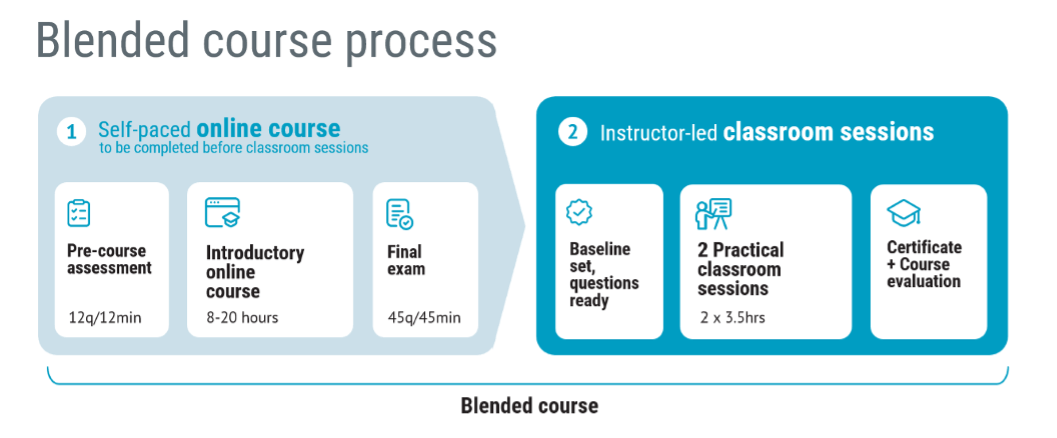
The topics were mainly design control and risk management for medical devices, and 99% of the participants were B2B customers. Typically, course participants spent 14–20 hours on the courses.
Learning is still changing and will continue to do so. E-learning and AI will play a big part in the future of learning solutions, but one thing is for sure: Learning will never go back to only traditional classroom courses.
At Medical Device HQ, we have seen better results and happier course participants since we took a step away from traditional classroom courses.
During the pandemic, we asked over 150 B2B course participants which type of course they preferred. At the time, over 80% of the respondents answered that they would prefer a blended (online + live virtual) course above other types of courses after the pandemic as well.
And now, two years after the end of the pandemic, we have evaluated the results of yet another survey. We asked over 800 medical device professionals who have attended professional B2B (business to business) training what kind of learning solutions they prefer now.
The results speak for themselves. Only 4% of participants continue to prefer traditional classroom courses, while the remaining 86% prefer at least some online component. 62% of the course participants answered that they prefer the type of blended course we specialise in (online + virtual classroom session) while 26% preferred an online course and a face-to-face classroom session.

The main reasons behind the course-taking preferences, as stated by the surveyed participants, were that they found the blended courses more engaging and interactive. Several common points that the majority stated were:
With demanding B2B customers that require a high return on the investment in training, one question would be how much people learned from a blended course compared to other types of courses. Not that shocking, but still very encouraging, were the results of asking the participants how much they had learned on the blended course compared to a traditional 2-day classroom course.
The typical time spent on the blended courses was 14–20 hours, thus the investment in time is similar to a 2-day traditional classroom course. If travel to a classroom course would be included in the equation, the time invested in blended learning would be shorter by comparison. Nevertheless, the 2-day classroom course was used as the benchmark to which blended learning was compared.
Out of all the people who took the blended courses on design control and risk management, a total of 85% thought they had learned more, or a lot more compared to a 2-day classroom course.

The comparison of the learning quality – blended vs. traditional
Measuring the results according to the participants’ answers is fairly easy, and when transferred to a visual representation, the results speak for themselves. The data shows that taking the blended course has proved to be more effective as opposed to traditional course-taking.
In the medical device industry, it is a regulatory requirement to evaluate the effectiveness of training actions taken. This means that when medical device companies are audited by external certification bodies or authorities, they may receive the question of how they know the training had the desired effect, and the companies must be able to show it. The blended format provided a good way of showing the effectiveness of the training by measuring results on a quiz before attending the training and after attending.

Pre-course assessment vs. final exam
The diagram shows results from a course and how it is reported to the client after the course to provide a record of the effectiveness of the training. Orange bars represent the pre-course assessment results and the blue bars represent the final exam results.
On average (n=236), the participant went from 53% on the pre-course assessment that was made up of 12 random questions from question banks, to 96% on the final exam with 45 questions from a question bank.
According to dr. George Siemens et al., it has been concluded that: “In the case of blended instruction, meaning both online, and any type of face-to-face time, the academic achievement of those students was higher than that of students who had either only online, or only face-to-face mode”
(Preparing for the Digital University: A Review of the History and Current State of Distance, Blended, and Online Learning, 2015, pp. 71).
The global pandemic (forcefully, but perhaps necessarily) opened a door to a new era of learning that could, ultimately, largely continue to improve future endeavours in pursuing knowledge. And we mean this not only in terms of education itself, but it could also be more cost and time-effective, as well as environmentally friendly as opposed to a traditional type of courses.
Medical Device HQ is a company that specialises in offering risk management and design control training to the medical industry. The courses (blended, online, and public) at MDHQ, held by highly educated and skilled professionals primarily focused on quality, are:
For over a decade now, MDHQ’s chief goal and vision has been to help as many as possible bring medical devices to market in the most efficient way.





Peter Sebelius is a highly esteemed trainer, consultant and entrepreneur in the medical device industry. He is a member of the Joint Working Group that is revising the ISO 13485 and ISO 14971 standards.
He has vast ‘hands on’ experience, having developed, amongst other things, a mechanical chest compression device and an ex vivo perfusion machine for lungs. He has received numerous awards including the Great Design Award and the title “This year’s specialist” by Veckans affärer.
Receive FREE templates and quarterly updates on upcoming courses that can help you in your career! Subscribe to our newsletter now.
When you submit this form, you will be sending personal information to medicaldevicehq.com. To comply with GDPR requirements, we need your consent to store and use the personal data you submit. Take a look at our Privacy policy for more details.
Special launch offer: 349 299 EUR for the online plan & 449 349 EUR for the online lifetime plan.