
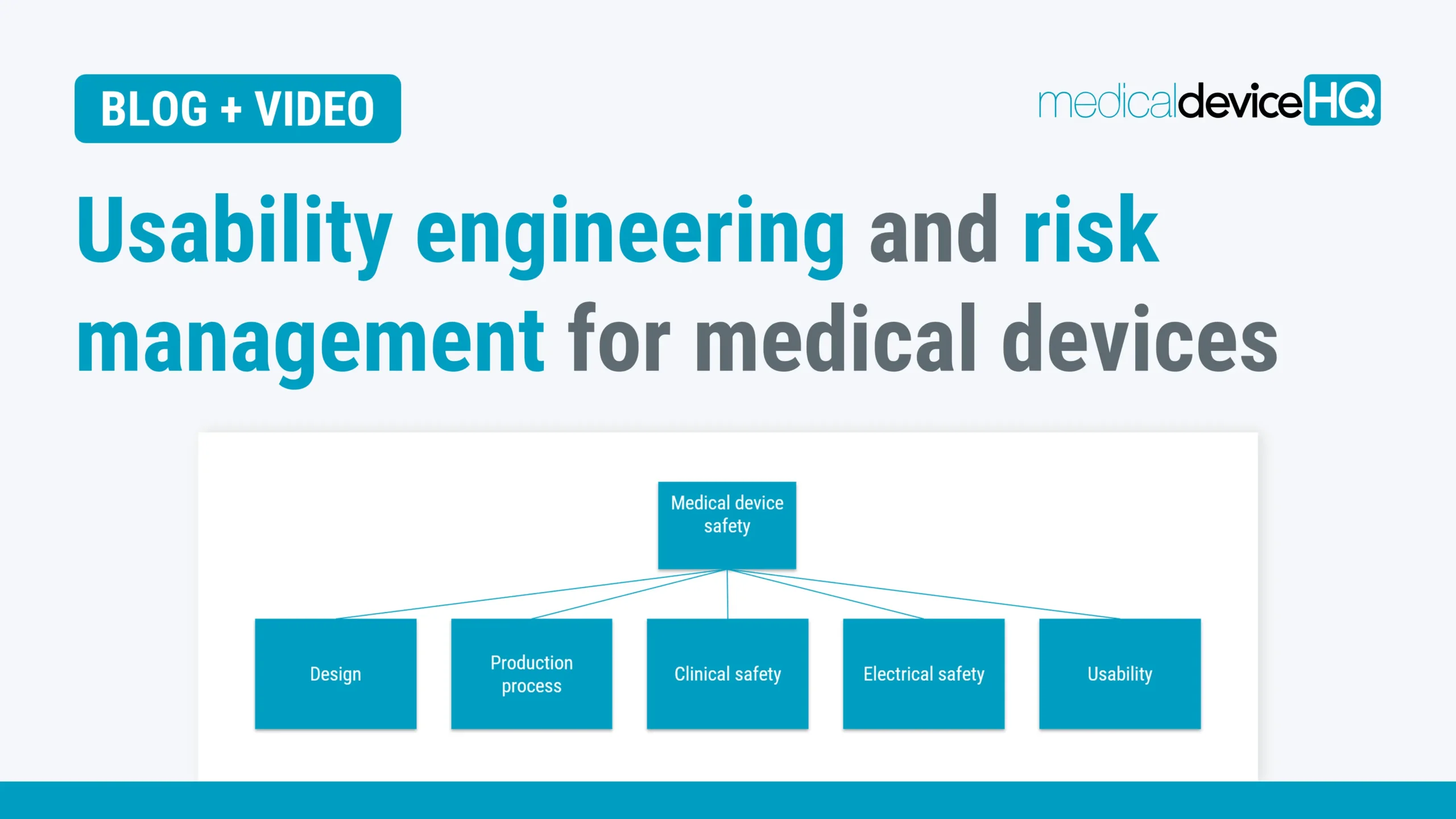
Usability engineering and risk management are connected in many ways, and all steps should be taken to ensure both safe and user-friendly medical devices for the end users.

Learn more about Medical Device Regulation (MDR) codes, as well as the EMDN codes which are the device-related codes needed under the MDR. They are also related to the notified bodies for class IIb devices, and manufacturers need to be aware of them because they are used when reporting to EUDAMED for all classes of devices.

This article explores the Covid-19 situation from a medical device risk management perspective and compares how the risks of Covid-19 would be addressed when applying ISO 14971 risk management compared to what governments have done.

It is important to know if a product is a medical device because this is what determines if the MDR applies to that product or not. Therefore, it has a major impact on how the device realisation is done as well as how the product should be approved for the European market.

This article aims to give tips and tricks so that medical device risk management work can be done more efficiently and effectively.

This article covers the typical document deliverables that you will have in a medical device product development project. Bear in mind that this is merely a simplified overview to help you understand the flow of information and the logic behind the process, as well as that different companies may have different approaches and use different terminology.

Summative evaluation is the final evaluation of your product’s user interface and confirmation that it does not result in an unacceptable risk to people. Essentially, it is the stage where you get to see if your whole usability engineering process was successful.

Providing basic documentation for all OTS software is expected. The documentation needs to consist of several points which answer the questions such as — what is it, what does it do, etc. Documentation serves as a piece of evidence.

Software system, item and units often cause both discussion and confusion. The blog post will try and clarify these within the terminology.

When it comes to the use of CVSS, it is by no means limited to non-medical software, which means that the answer to the question from the title is yes, it can be used for medical device software too.

Take a look at our online Risk Management course on ISO 14971:2019 and online Design Control for Medical Devices course.
These courses are taken by both competent authorities, notified bodies and medical device manufacturers and distributors.
Once you have submitted the form, you will be automatically taken to your cart where the e-book and 100% discount will be applied. Go through checkout to get the free e-book.
Press here to subscribe to our newsletter and get your free e-book
Special launch offer: 349 299 EUR for the online plan & 449 349 EUR for the online lifetime plan.