Revalidation requirements: Medical device process validation
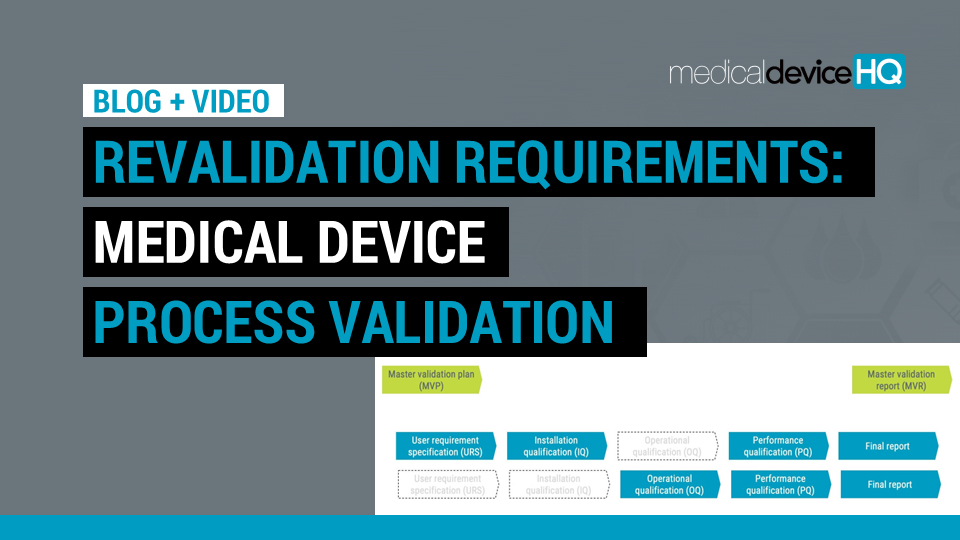
Maintaining a validated state for your manufacturing processes is crucial to ensure product quality and regulatory compliance.
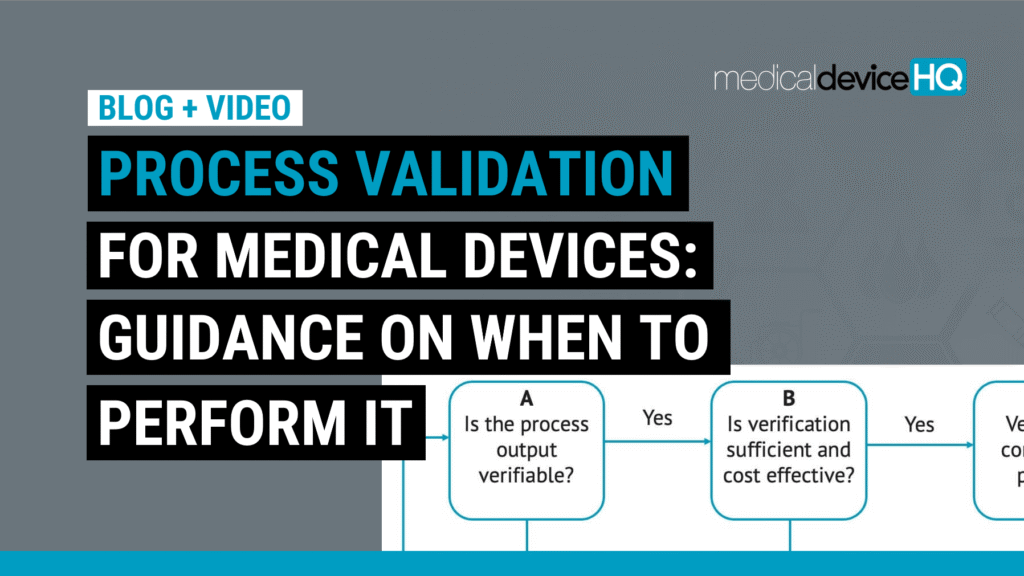
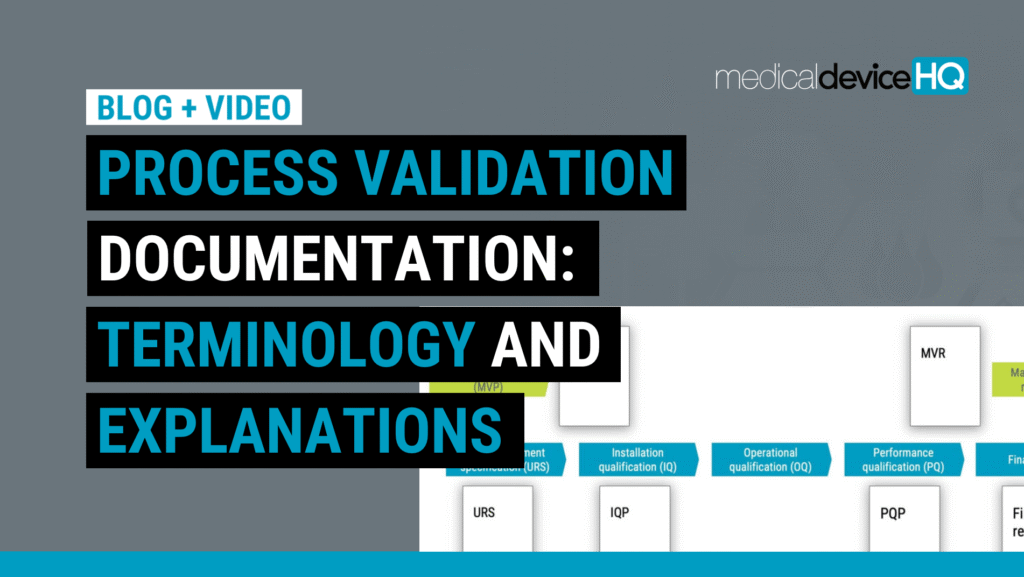
This article discusses when processes need to be revalidated and when certain validation steps could potentially be skipped.