
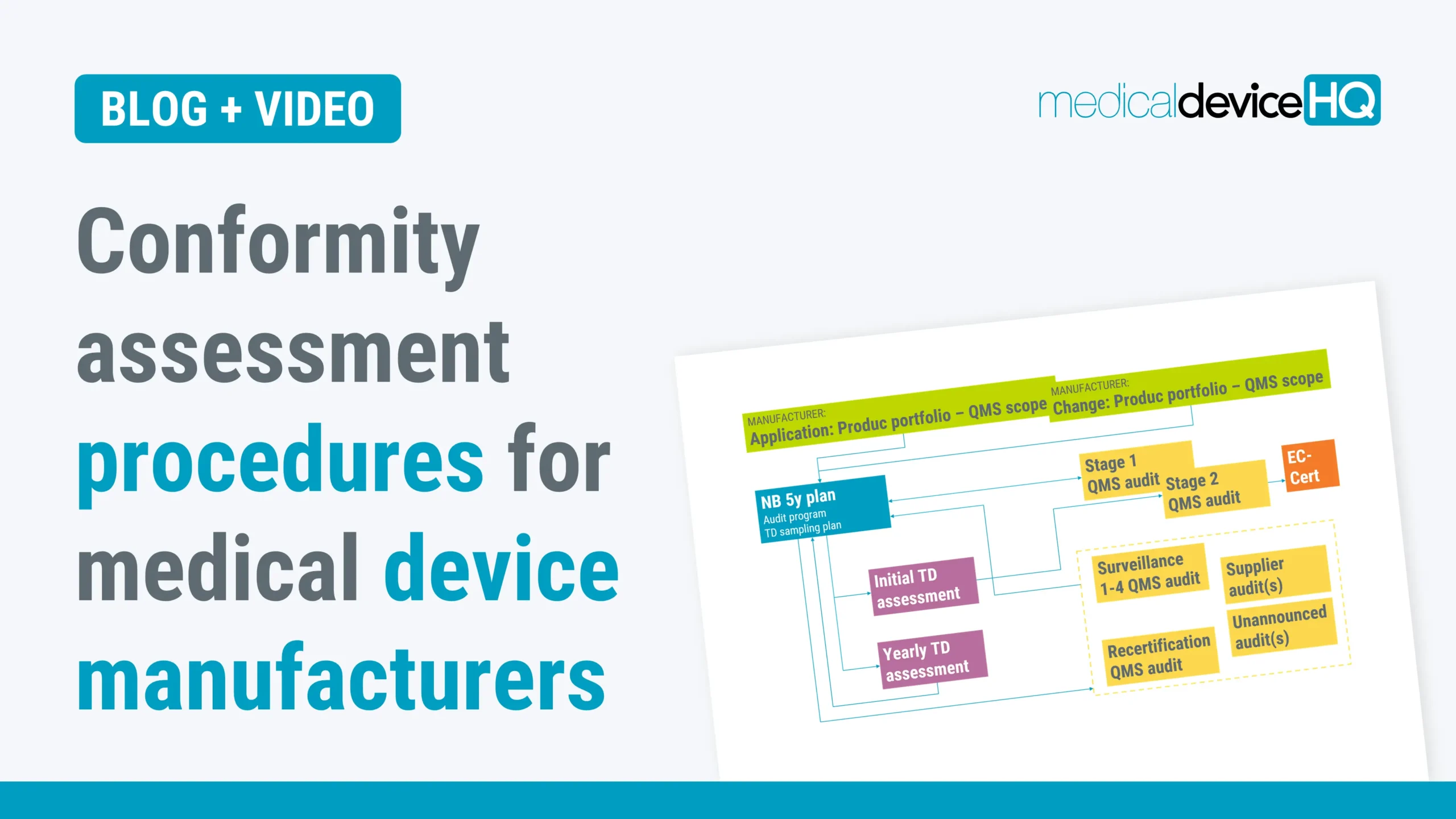
Most manufacturers consider the notified body conformity assessment the most challenging part of the CE marking process. This article outlines the general setup of the conformity assessment procedure for medical device manufacturers.

Find the ISO 13485:2016 standard for free online on ANSI’s website. Please follow the steps in this article to access the standard.

GCP is an international quality standard that governments can transpose into regulations that decide how clinical trials involving human subjects must be managed. Understanding the GCP principles allows for a successful setup and management of clinical studies and collecting data that can stand up to regulatory scrutiny.

The ISO 14155 standard has undergone some changes in its 2020 edition, mainly in order to ensure that the standard continues to keep its global acceptance by regulatory authorities. This article will present the main changes compared to the previous version of the standard.

Every stakeholder within the MDR has their own roles to fulfil, and in the medical device industry, it is of great importance to know exactly what those roles are, as is to know the difference between the stakeholders and economic operators.
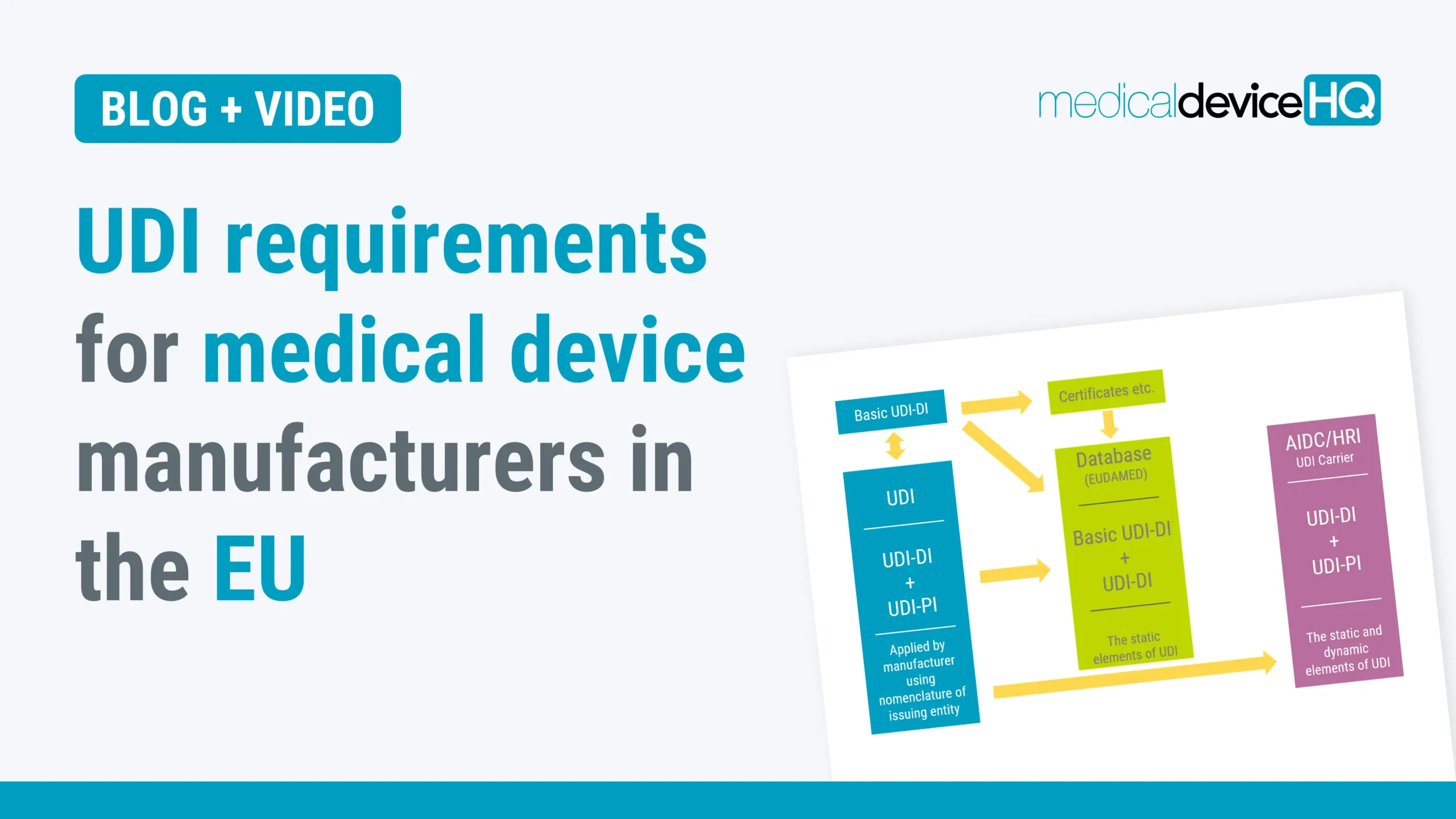
This article provides a summary of the Unique Device Identifier (UDI) requirements. UDI will be applicable to all manufacturers of medical devices in the EU. Please note, the UDI requirements in the EU are not identical to the UDI requirements of other markets, like the US market.

Clinical research is necessary to develop new treatments for different diseases and conditions that occur in humans. To develop these new treatments, clinical trials have to be performed to get proof that the new treatment works and that it is safe.

This article focuses on the purpose and scope of essential SOPs (Standard operating procedures) and controlling documents in a QMS. It also covers how and why you should write a thought-through purpose for your SOP or controlling document and how this impacts efficiency.

In June 2020, the IEC 62366-1 standard underwent some updates – Let us look at the eight most important changes in the AMD1:2020 in order of importance.
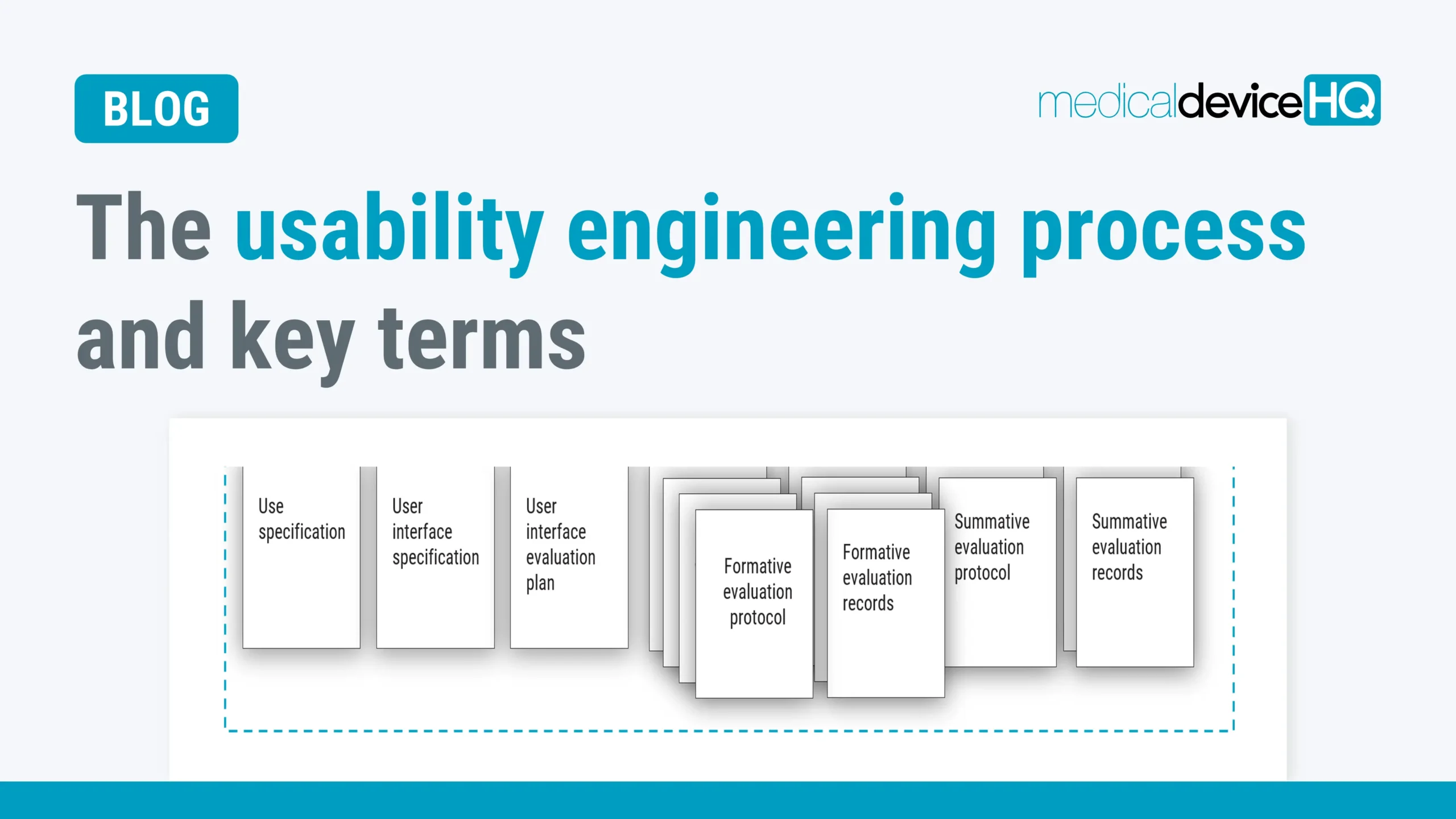
Usability engineering is about understanding the users of a device, their tasks, the environment they will use it in, and designing a great user interface. Having a good understanding of the process and the key terms helps you develop safe medical devices.

Take a look at our online Risk Management course on ISO 14971:2019 and online Design Control for Medical Devices course.
These courses are taken by both competent authorities, notified bodies and medical device manufacturers and distributors.
Once you have submitted the form, you will be automatically taken to your cart where the e-book and 100% discount will be applied. Go through checkout to get the free e-book.
Press here to subscribe to our newsletter and get your free e-book
Special launch offer: 349 299 EUR for the online plan & 449 349 EUR for the online lifetime plan.