

Post-market surveillance reporting requirements are different depending on the classification of the devices the PMS system covers. This article provides an overview of PMS reporting based on medical device classification.

Dealing with post-market surveillance (PMS) is important for medical devices on the EU market. It is also one of the focus areas of the notified bodies. This makes it essential for all manufacturers and others working within the medical device industry.

Comparing the least expensive version with the most expensive ones will result in a price difference of 1400%. The price at the Estonian Centre for Standardisation is significantly lower than the others. Find out where to buy and download the standard.

Performing risk evaluation is perhaps the easiest part of risk management. Yet, it is important to know how to do it properly to perform risk management successfully.

One of the most underestimated aspects of being a usability engineer is the mindset. To maybe no surprise, “mindset” is not mentioned in the IEC 62366-1 standard in usability engineering.
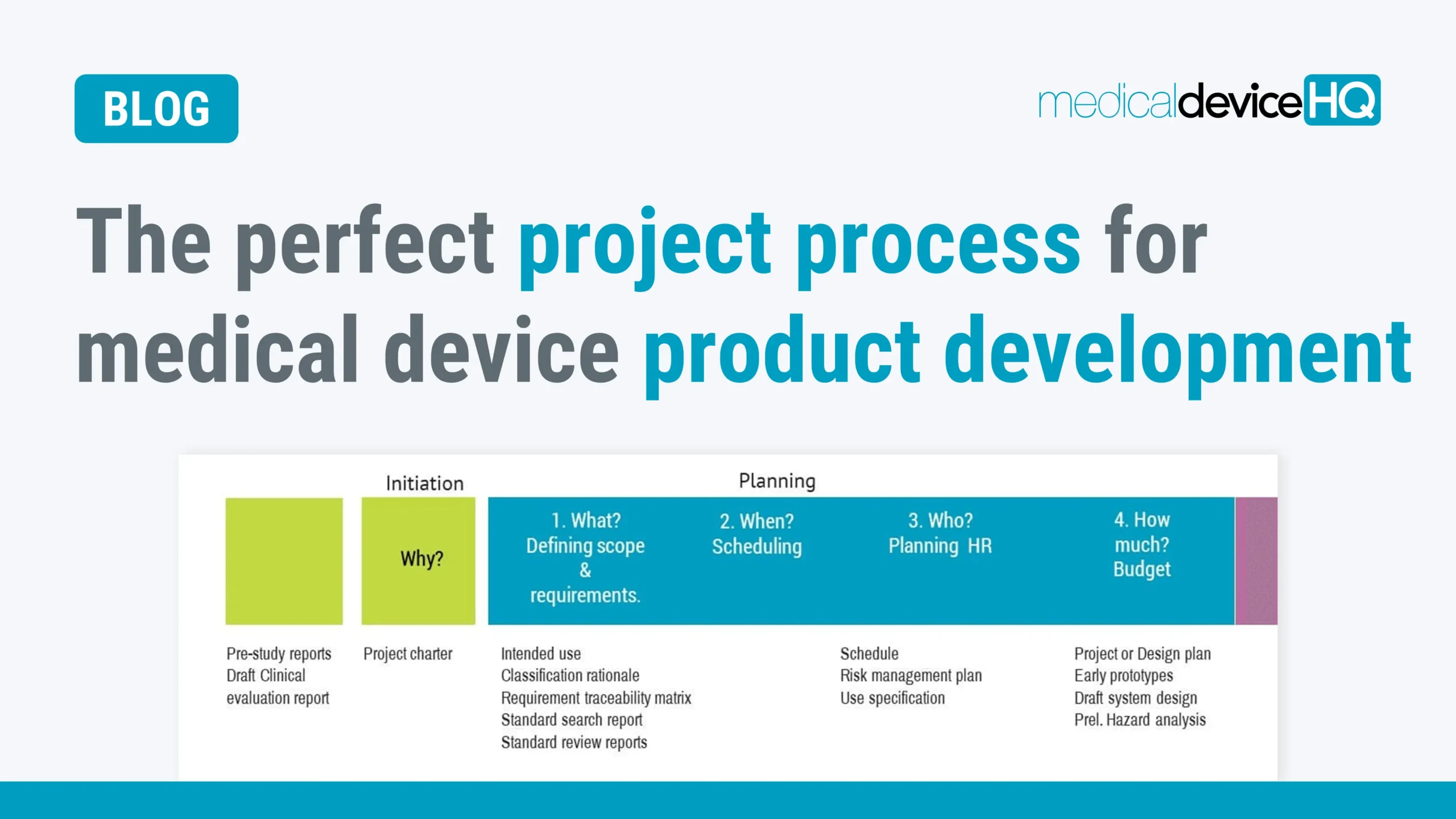
A well-recognized project process can allow you to exercise control over projects, especially in a multi-project environment. This is key in developing products in a short time and at a low cost while still meeting regulatory requirements.

Anyone involved with clinical investigation should have a copy of the ISO 14155:2020 standard because it contains everything that is needed on good clinical practice, GCP. But it can be expensive if you buy it in the wrong place.
In this article, you will learn where to buy the standard for a fraction of the price compared to major outlets.

This article provides an in-depth explanation of EUDAMED logins. You may want to know how to complete all the steps to register as an actor within the database, or how to search for the information you need.

The EUDAMED database enhances traceability, cooperation, and transparency regarding medical devices in the EU. The database is split up in six modules with specific purposes. This article provides an overview of the areas that these modules covers.

Take a look at our online Risk Management course on ISO 14971:2019 and online Design Control for Medical Devices course.
These courses are taken by both competent authorities, notified bodies and medical device manufacturers and distributors.
Once you have submitted the form, you will be automatically taken to your cart where the e-book and 100% discount will be applied. Go through checkout to get the free e-book.
Press here to subscribe to our newsletter and get your free e-book
Special launch offer: 349 299 EUR for the online plan & 449 349 EUR for the online lifetime plan.