

Among other things, this illustrated guide provides a useful overview of quality management for medical devices and ISO 13485, clarifies terminology used in the ISO 13485 standard that is defined elsewhere, and addresses common misconceptions.


As the final part of this series, this article focuses on categorising risks based on the different life-cycle phases of a medical device in order to efficiently address them to avoid harm. Not all medical device manufacturers segment their documentation into three distinct documents but risk association with life-cycle phases is common in the industry.

Part 2 of this series of three articles investigates the dangers of dividing risk management documentation in general or according to Team NB’s recommendation. By using a heart-lung machine as an example, this article will present a comprehensive approach to risk control measures in medical device risk management.

This first article in a series of three will be discussing the dangers of dividing risk management documentation according to Team NB’s recommendation. When performing risk management according to ISO 14971, a lot of medical device companies split up risk management documentation into “design risk assessment”, “process risk assessment” and “use risk assessment”. Not only is this common practice, but it is also recommended by Team NB.

This article covers the responsibilities of the sponsor – one of the most important stakeholders in a clinical investigation. Some of the main responsibilities include The main sponsor responsibilities are: clinical quality management, communication with regulatory authorities, clinical investigation planning and conduct, and outsourcing duties and functions.
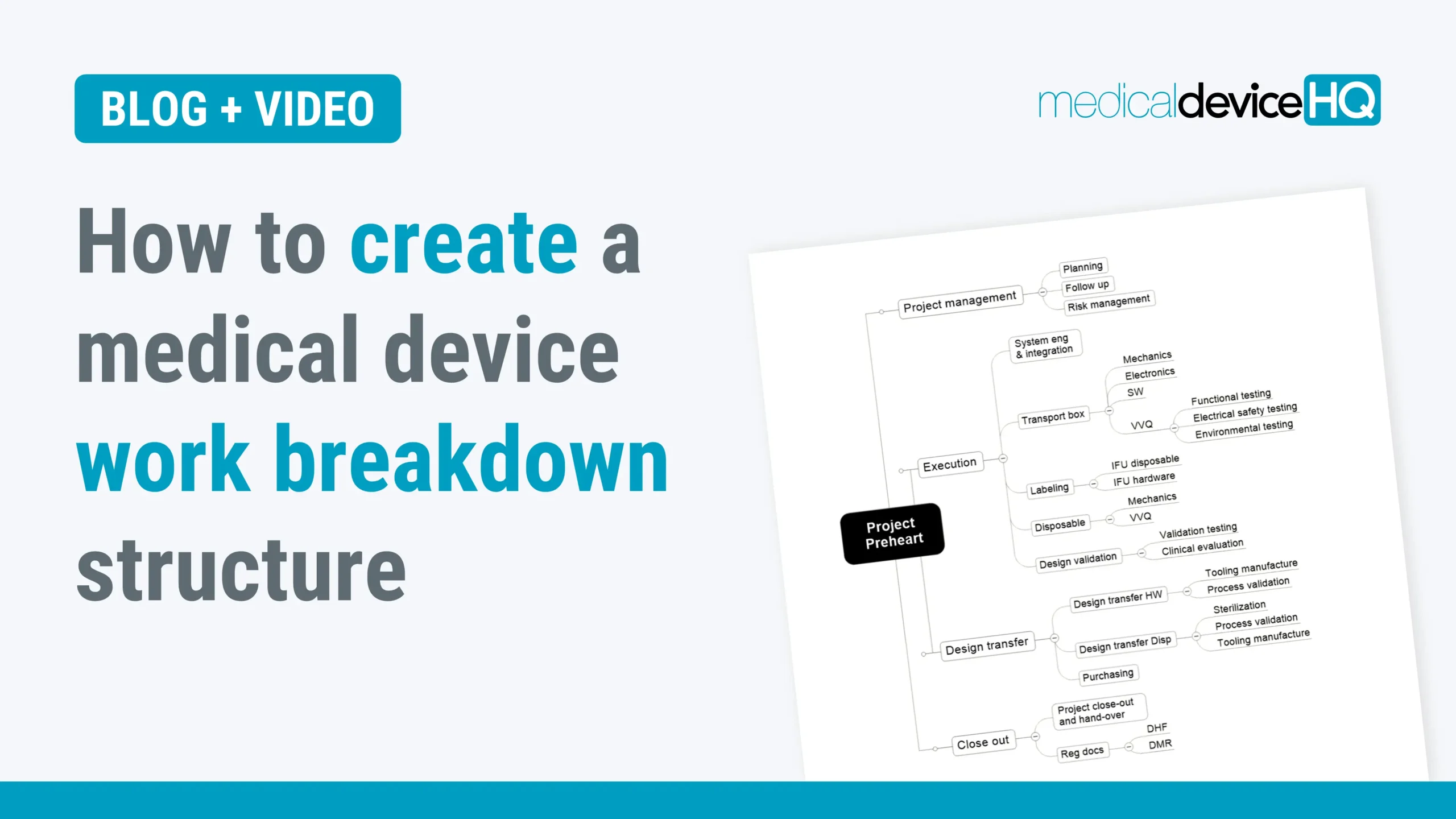
A work breakdown structure is a very useful tool for any project manager, yet many do not know what it is. This article provides an overview of how a medical device work breakdown structure (WBS) is created and its key benefits.

To achieve the purpose of the manufacturer’s PMS system, the manufacturer must have a PMS plan. This is also stated in Article 84 of the MDR. But article 84 basically only references Annex III, which is the annex that describes the requirements on the technical documentation of the PMS.

Take a look at our online Risk Management course on ISO 14971:2019 and online Design Control for Medical Devices course.
These courses are taken by both competent authorities, notified bodies and medical device manufacturers and distributors.
Once you have submitted the form, you will be automatically taken to your cart where the e-book and 100% discount will be applied. Go through checkout to get the free e-book.
Press here to subscribe to our newsletter and get your free e-book
Special launch offer: 349 299 EUR for the online plan & 449 349 EUR for the online lifetime plan.