
This article will introduce you to the insulation requirements as identified in the IEC 60601-1. Insulation is one of the methods used to protect patients and operators from electrical hazards. By managing your insulation design in a proactive way, you ensure that the necessary safety requirements for your medical device are met.
Find out more about the IEC 60601-1 insulation requirements for electrical medical devices in the short video below.
In general, medical electrical equipment should have two means of protection (MOP) to prevent leakage current from applied parts and accessible parts. Essentially, there should be a basic insulation and a supplementary insulation to provide sufficient MOP.
Each MOP is categorised either as operator protection (MOOP) or patient protection (MOPP). The requirements for patient protection are intended to provide a higher level of protection than the requirements for operator protection.

Insulation can be provided in various forms; solid insulation, creepage distance and air clearance, as well as by components. When considering your insulation design, expect short circuit of any insulation that does not meet the requirements for a means of protection.
A component will be exempt from testing if you have identified it as ’critical’ and demonstrated that it complies with a relevant component standard. Creepage and clearance is measured physically or in a CAD program. The measured distances are compared to the minimum requirements defined by the tables in the standard.
Let’s move on to solid insulation. The dielectric strength of solid insulation is tested by applying a high voltage across the insulation. If the peak working voltage is greater than 71 V, then there are additional requirements to the physical construction. Solid insulation should have a minimum thickness of 0.4 mm unless there are alternative requirements.
For insulation other than wire insulation, its effectiveness must last for the duration of the expected life service of the medical device. It should also take into consideration environmental stresses such as mechanical wear and tear, heat and moisture.
A secondary circuit is separated from the mains part by at least one means of protection and subsequently has reduced requirements. A secondary circuit can also be supplied from a battery.
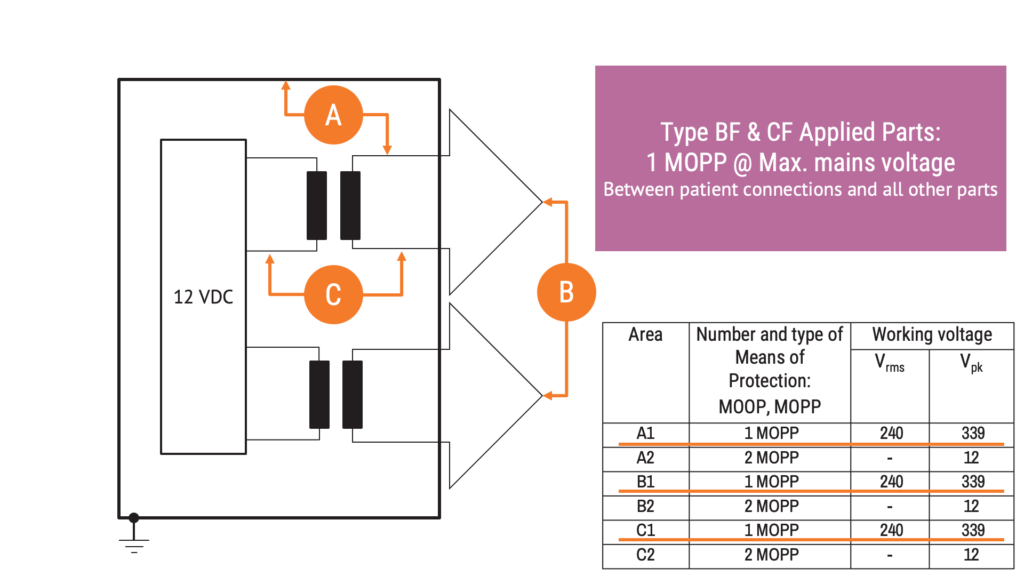
The image above shows F-type applied parts. All patient connections should be separated from all other parts by means equivalent to one means of patient protection for a working voltage equal to the maximum mains voltage. This requirement can be stricter than the general requirement of 2 MOPP for a working voltage equal to the voltage in secondary circuits.

The example shown in the diagram above could be an infusion pump, which can be classified as a type B applied part because the patient connection through the fluid column is not intended to deliver electrical energy or an electrophysiological signal to or from the patient. Here, the patient connection should be separated from metal accessible parts.
If you want to know more about the 60601 standard and safety for electrical devices, take a look at our online course Introduction to Safety for Medical Devices and IEC 60601. This comprehensive course has in-depth information and quizzes to test your knowledge and understanding. At the end of the course you will also receive a course certificate, which many auditors will be looking for.
Our online courses are frequently taken by competent authorities, notified bodies and medical device manufacturers and distributors.

Claus Rømer Andersen is an accomplished trainer, consultant and facilitator in the medical device industry. With a background in electrical engineering, he has worked with regulatory navigation, approval management, device testing throughout his whole career.
He is recognized as having a pragmatic and solution oriented approach to helping development teams focus on relevant issues throughout the entire product life-cycle.
Receive FREE templates and quarterly updates on upcoming courses that can help you in your career! Subscribe to our newsletter now.
When you submit this form, you will be sending personal information to medicaldevicehq.com. To comply with GDPR requirements, we need your consent to store and use the personal data you submit. Take a look at our Privacy policy for more details.
Important! Please be advised that there will be scheduled downtime across our platforms from 13:00 CET Apr 26th to no later than 16.00 CET Apr 28th. During this period you will not be able to access the website or your account. For more information, please contact us at support@medicaldevicehq.com